Summary
- Opdivo had positive trial results for esophageal cancer, melanoma, and non-small cell lung cancer.
- This should reassure investors that sales will continue to rise.
- Celgene shareholders benefit when BMY stock rises.
Bristol-Myers Squibb (BMY) is a large-cap ($82 billion), diverse pharmaceutical company that has been in the news due to its planned merger with Celgene (CELG). Bristol-Myers Squibb derives about two-thirds of its revenue from two of its drugs, the cancer therapy Opdivo and the anticoagulant Eliquis. A series of Opdivo trial results released at ESMO over the weekend reinforce the proposition that Bristol-Myers Squibb has been through a period of being undervalued by the market. That, in turn, implies that Celgene has been undervalued, as investors will receive $50 per share plus one Bristol-Myers Squibb share plus a CVR (contingency value right).
Bristol-Myers Squibb's 52-week low of $42.48 was reached largely on the hypothesis that new drug revenues would not make up for drugs running into generic or biosimilar competition, including the portfolio to be acquired with Celgene. The ESMO (European Society for Medical Oncology) presentations show the strength of the Opdivo and Yervoy franchises. That does not force the market to return to the 52-week high of $63.69, but it makes it more likely.
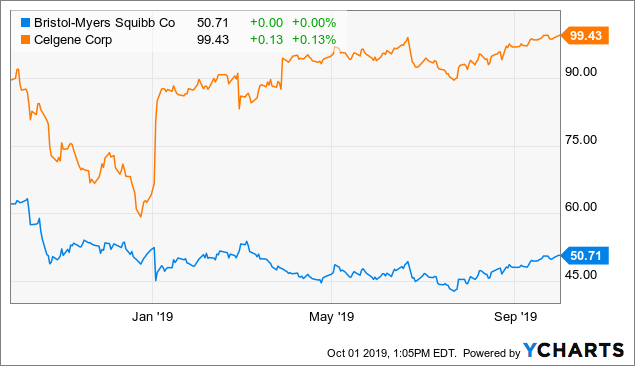 Data by YCharts
Data by YChartsOpdivo for esophageal cancer
On September 30, 2019, Bristol-Myers Squibb and Ono Pharmaceutical (OTC:OPHLF) announced results from the Phase 3 ATTRACTION-3 trial evaluating Opdivo (nivolumab) versus chemotherapy (docetaxel or paclitaxel) for unresectable advanced or recurrent ESCC (esophageal squamous cell carcinoma) second-line patients whose cancers were refractory or intolerant to combination therapy with fluoropyrimidine and platinum-based drugs. The primary endpoint was OS (overall survival). Opdivo demonstrated a statistically significant improvement over chemotherapy, with a 23% reduction in risk of death and a 2.5-month improvement in median OS compared to patients treated with chemotherapy. The safety profile of Opdivo in this trial was consistent with previously reported studies.
Second-line esophageal cancer is both common and difficult to treat, with a low survival rate, so having Opdivo available as a therapy will be welcomed and should produce significant revenue, presuming the indication is approved by the FDA and other regulatory agencies.
Immune cell. Source: bms.com















